
The Manufacturing Capability
Chemman is a world class, ultramodern Greenfield Pharmaceutical facility to serve mankind with niche formulations. This facility is built on 10 acre of land near Mandideep Industrial Area close to City of Lakes-Bhopal in the central Indian state of Madhya Pradesh.
The facility has been setup keeping in mind global regulatory requirements such USFDA, EU GMP, UK MHRA and PICs. The facility has a manufacturing line for, Biosimilar including MABs DP filling with Fundamix®.

R&D Capability
The company has expertise in development of Biological products that are complex to manufacture and handle, but have a greater acceptance in global markets.
Chemman has allocated adequate resources in R&D to ensure that it will emerge as one of the best R&D driven pharmaceutical organization in the near term horizon to have a healthy portfolio and strong pipeline for Global markets.
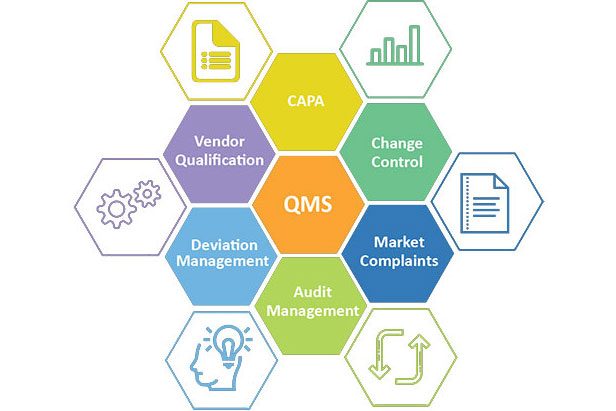
Quality Systems
The quality management system at Chemman include capabilities to perform Stability Studies as per ICH guidelines for stability Zone IV b and Zone II, catering to the requirements of almost all geographic regions. The facility has the complete critical analytical equipment as per 21 CFR part 11 compliance with stringent GDP/GLP practices across all the analysis.
All QMS activities are controlled by QMS software that complies with 21CFR part 11 built on SAP B1 platform to meet international quality standards in the field of health care products.
